
Mandar Karhade, MD PhD MPH
- Chief Artificial Intelligence Officer at Quench
- Sr. Director, AI/ML & Data Science at Avalere Health
- Lead Data Scientist at McKesson | Ontada
- Sr. Research Scientist at MD Anderson Cancer Center

- Chief Artificial Intelligence Officer at Quench
- Sr. Director, AI/ML & Data Science at Avalere Health
- Lead Data Scientist at McKesson | Ontada
- Sr. Research Scientist at MD Anderson Cancer Center

This afternoon, a hands-on session where participants experienced AI tools as part of an AI-Enabled Medical Device Overview was led by @Mandar Karhade, MD PhD. The workshop on AI/ML Enabled Medical Devices from a Quality & Regulatory Perspective kicks off MedCon 2024 hosted the AFDO/RAPS Healthcare Products Collaborative and co-sponsored by the FDA. hashtag#FDA
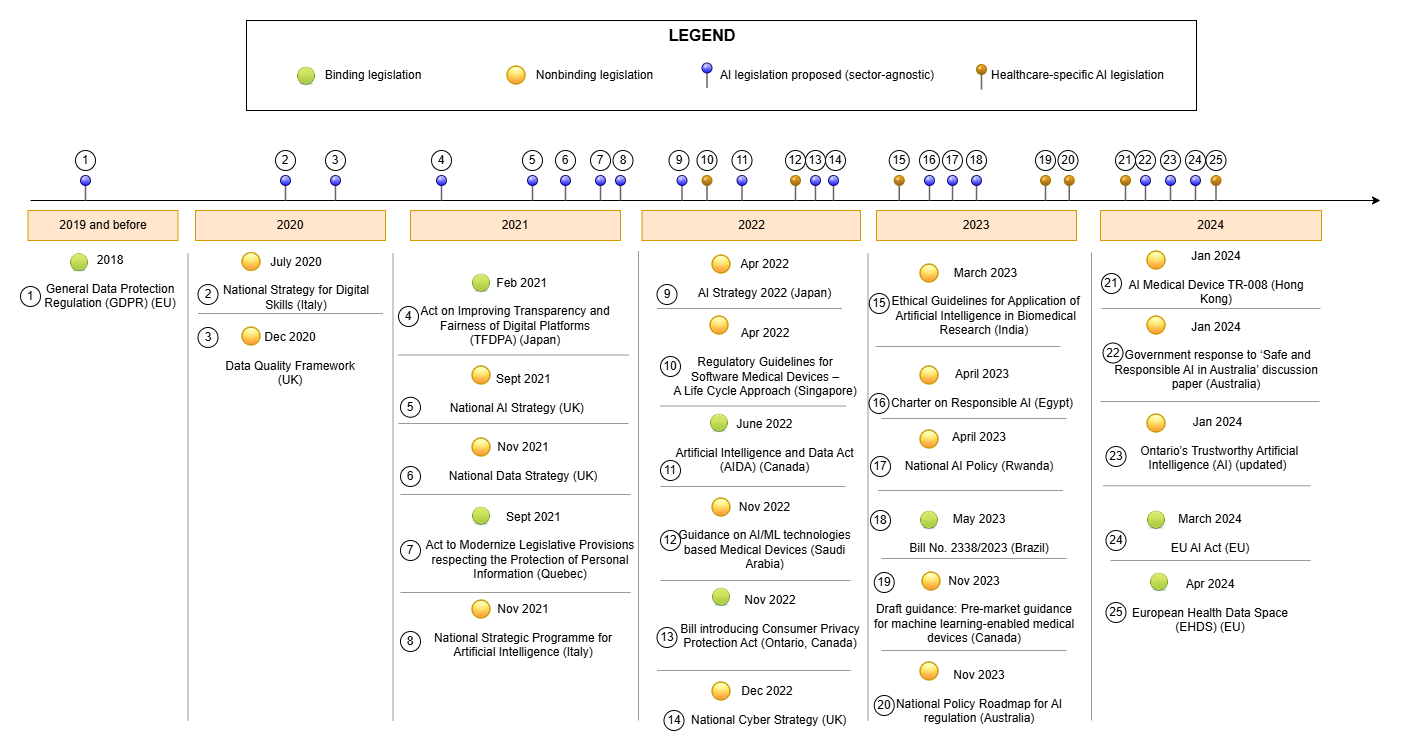
Artificial Intelligence (AI) is being adopted across the world and promises a new revolution in healthcare. While AI-enabled medical devices in North America dominate 42.3% of the global market, the use of AI-enabled medical devices in other countries is still a story waiting to be unfolded. We aim to delve deeper into global regulatory approaches towards AI use in healthcare, with a focus on how common themes are emerging globally. We compare these themes to the World Health Organization's (WHO) regulatory considerations and principles on ethical use of AI for healthcare applications. Our work seeks to take a global perspective on AI policy by analyzing 14 legal jurisdictions including countries representative of various regions in the world (North America, South America, South East Asia, Middle East, Africa, Australia, and the Asia-Pacific). Our eventual goal is to foster a global conversation on the ethical use of AI in healthcare and the regulations that will guide it. We propose solutions to promote international harmonization of AI regulations and examine the requirements for regulating generative AI, using China and Singapore as examples of countries with well-developed policies in this area.. hashtag#FDA

As the number of Artificial Intelligence and Machine Learning (AI/ML)-enabled medical devices cleared by FDA approaches 1,000, the challenges associated with incorporating this technology are still significant. The level of understanding around AI/ML fundamental concepts in the industry is still very low; regulatory frameworks are not yet designed to take advantage of the greatest benefits of AI/ML, and there are many barriers to trust in these technologies to safely and effectively diagnose and treat patients (e.g., bias, transparency, explainability). This daylong workshop aligns attendees on vocabulary and concepts and includes a hands-on session that takes a simple AI algorithm and moves it through the training, testing, and tuning process. The workshop will also include: An overview of current and future regulatory trends in AI/ML-enabled medical devices. The impact of AI/ML on Quality and Regulatory functions. Technology, intended use, and trustworthiness challenges that the medical device industry and regulators must address. A case study walking attendees through development, submission, clearance, and deployment of an AI/ML-enabled medical device